38 labels on repackaged bulk food for resale must include
Good manufacturing practices guide for drug products (GUI-0001) About this document 1. Purpose. This guide is for people who work with drugs as: . fabricators; packagers; labellers; testers; distributors; importers; wholesalers; It will help you understand and comply with Part C, Division 2 of the Food and Drug Regulations (the Regulations), which is about good manufacturing practices (GMP). You can find definitions to terms used in this guide … Join LiveJournal must contain at least 4 different symbols; at least 1 number, 1 uppercase and 1 lowercase letter; not based on your username or email address. Learn more here. Password confirm. Birthday: Required by law. Only month and day are displayed by default. I am: By creating an account on LiveJournal, you agree to our User Agreement. Create account . Or you can use social network …
Pesticide Labeling Questions & Answers | US EPA 13/10/2022 · If labels do not specifically state that they can be used in food storage facilities or food processing plants, can these rodenticides be used under 21 CFR 110.35(c), which states in part " The use of insecticides or rodenticides is permitted only under precautions and restrictions that will protect against the contamination of food, food-contact surfaces, and food-packaging …
Labels on repackaged bulk food for resale must include
(PDF) Ansel's Pharmaceutical Dosage Forms and Drug Extemporaneous compounding takes place in community and hospital pharmacies. There are usually specialist compounding pharmacies in major towns and cities, but any pharmacy may undertake compounding as long as they have appropriate facilities according to state-based legislation (e.g. allocated clean bench, specific compounding equipment). Code of Laws - Title 40 - Chapter 43 - South Carolina General … SECTION 40-43-10. Short title; purpose of chapter; severability. This chapter may be cited as the "South Carolina Pharmacy Practice Act". The purpose of this chapter is to promote, preserve, and protect the public health, safety, and welfare by and through the effective control and regulation of the practice of pharmacy; the licensure of pharmacists; the licensure, permitting, control, and ... Reference Manual for the WHMIS Requirements of the Hazardous … Accordingly, instructions that include a recommendation of a hazardous product do not constitute an advertising violation, unless the hazardous product is being promoted and is made available for sale at the same time. Controlled product: A "controlled product" is any product, material or substance that meets any of the criteria listed in Part IV of the Controlled Products …
Labels on repackaged bulk food for resale must include. Industry Guide for the labelling of cosmetics - Canada.ca If the manufacturer chooses to include such a declaration on the inner label, it must not be false or misleading to the consumer. 5.2 Products that have an inner label only Products that have an inner label only must meet the same labelling requirements as those for the outer label of products that have both an outer and inner label ( see section 5.1.1,"Outer label requirements" ). Chapter 545 - Liquor Control Act - Connecticut General Assembly Lease providing for reduction of rental if city “should go no license” held to include Volstead act. 98 C. 751. Note given for purchase price of interest in saloon or intoxicating liquors is void between parties; if such note is given for purchase price of place reputed to be a place where such liquors were sold, holder may recover by showing reputation was not founded on fact. 104 C. … General Chapters: <1178> GOOD REPACKAGING PRACTICES A REPACKAGER is one who purchases and removes a drug product from the manufacturer's market container or bulk dosage container and places the product into a different container for distribution for human or animal use. A repackager may or may not take ownership from the manufacturer. A repackager is engaged in the repackaging of drugs (see also Packaging … HEALTH AND SAFETY CODE CHAPTER 431. TEXAS FOOD, DRUG, … (a) Except as provided by Section 431.2211, a food manufacturer, food wholesaler, or warehouse operator in this state must apply for and obtain from the department every two years a license for each place of business that the food manufacturer, food wholesaler, or warehouse operator operates in this state. The food manufacturer, food wholesaler, or warehouse operator must …
Reference Manual for the WHMIS Requirements of the Hazardous … Accordingly, instructions that include a recommendation of a hazardous product do not constitute an advertising violation, unless the hazardous product is being promoted and is made available for sale at the same time. Controlled product: A "controlled product" is any product, material or substance that meets any of the criteria listed in Part IV of the Controlled Products … Code of Laws - Title 40 - Chapter 43 - South Carolina General … SECTION 40-43-10. Short title; purpose of chapter; severability. This chapter may be cited as the "South Carolina Pharmacy Practice Act". The purpose of this chapter is to promote, preserve, and protect the public health, safety, and welfare by and through the effective control and regulation of the practice of pharmacy; the licensure of pharmacists; the licensure, permitting, control, and ... (PDF) Ansel's Pharmaceutical Dosage Forms and Drug Extemporaneous compounding takes place in community and hospital pharmacies. There are usually specialist compounding pharmacies in major towns and cities, but any pharmacy may undertake compounding as long as they have appropriate facilities according to state-based legislation (e.g. allocated clean bench, specific compounding equipment).




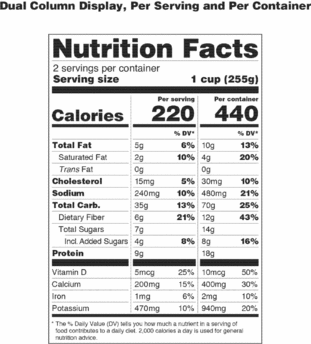
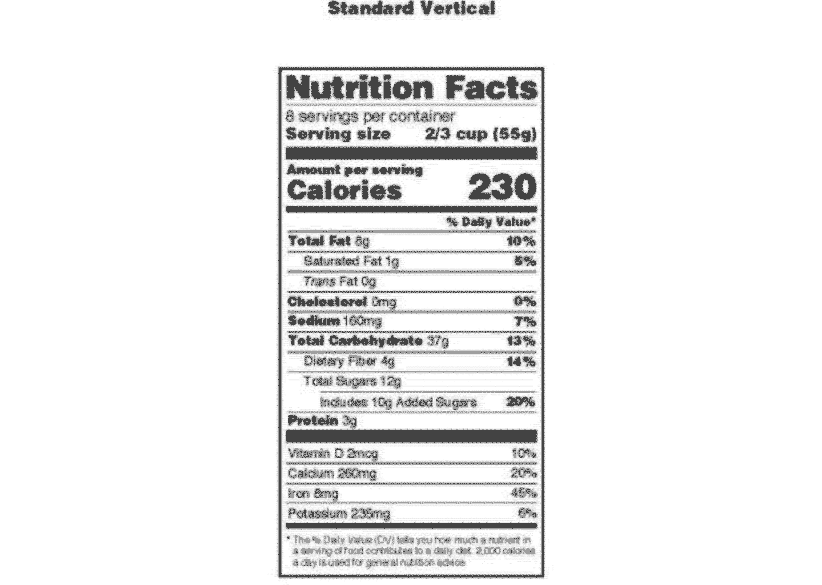


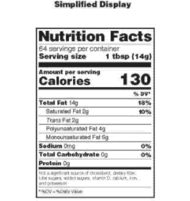


















Post a Comment for "38 labels on repackaged bulk food for resale must include"